Abstract
The ability to achieve a cervical-cranial-facial postural system that allows one to move and breathe in all three planes with balanced neuromuscular function and position can be significantly influenced by certain anatomical and physiological characteristics within it. One of these factors involves how one postures with their tongue in relationship to their palate and its correlation to craniofacial system development. This includes such variables as the size of different parts of the cranium (particularly the maxilla), the anatomical relationships between them, and the interrelated sensory-motor behavior that learns from its anatomy as well as directs its inhabited structure. Furthermore, a major factor driving craniofacial growth and function is nutritional in nature that interacts at the epigenetic level. This article aims to connect these postural, developmental, and nutritional variables together as well as demonstrate potential points of intervention along the continuum. Such treatment opportunities serve to both prevent as well as restore structural and functional relationships of the cervical-cranial-facial postural system.
Connecting Postural Dysfunction and Craniofacial Development
The behavior of habitually posturing with the tongue on the palate has a number of important benefits to the postural system. First, provided one has enough nasopharyngeal space, it facilitates nasal vs. mouth breathing. Nasal breathing serves to warm, humidify, and filter the air for sanitization and maximizing gas exchange capacity. It also facilitates nitric oxide production in the sinuses which stimulates vasodilation in the lungs for efficient gas diffusion as well as act as a potent anti-bacterial and anti-viral agent. On the other hand, posturing with the mouth open lowers and retrudes the tongue, mandible, and hyoid. This can occlude the oropharyngeal airway space resulting in compensatory posterior cranial rotation to further open the airway. As a result of this re-posturing, in order to level the ocular plane due to the upper cervical and cranial extension, the neck assumes a more forward and reduced cervical lordotic posture. (Solow, Ovesen, Nielsen, Wildschiodtz, & Tallgren, 1993) The mandibular and tongue retrusion that occurs with a mouth breathing posture are associated with the hyoid moving posteriorly and inferiorly which can further occlude the airway. Forward head posture helps to restore the hyoid position and further increase airway patency. (Meiyappan, Tamizharasi, Senthilkumar, & Janardhanan, 2015) Mouth breathing also facilitates increased accessory muscle breathing use and is associated with shoulder and pelvic asymmetries. It is correlated to decreased nocturnal genioglossus, tensor palatine, upper airway dilator, and intercostal activity which can facilitate airway obstruction. (Uhlig, Marchesi, Duarte, & Araújo, 2015) Furthermore, forward head posture lengthens and narrows the airway making it more prone to collapse during inspiration thus encouraging apnea behaviors. (Yeol et al., 2013)
There appears to be a functional parallel between the diaphragm and the tongue. The diaphragm serves to pump air as well as lymphatic fluids throughout the body via its oscillatory downward contraction during inhalation and upward elevation during exhalation coupled with the subsequent pause before the next breath. When the diaphragm is at its highest point it is also maximally in apposition to the rib cage. This is referred to as the zone of apposition and is taught in detail via the Postural Restoration Institute (“Postural Restoration Institute,” 2013) as a crucial element to restoring breathing and postural function of the body. Essentially, if an individual does not possess an appropriate zone of apposition the diaphragm will not be able to fully relax to allow adequate inhalation and exhalation function. It can often get stuck in a contracted, downward, and poorly opposed position against the rib cage that will tend to leave the individual in more of an inhaled state of function. Due to the diaphragm’s anatomical connection with the psoas, the increased tension in a poorly apposed diaphragm may extend into the psoas as well. This can contribute to increased lordosis in the lumbar spine, elevation of the anterior inferior aspect of the rib cage (external rotation), and a myriad of other postural compensations throughout the entire body.
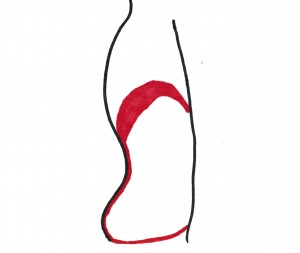 Respiratory and Pelvic Diaphragms |
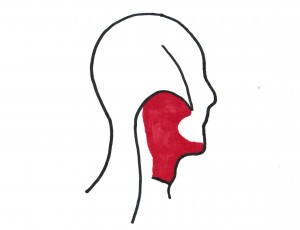 Cranial Diaphragm (Figure adapted from the Postural Restoration Institute) |
The rest position of the tongue against the palate is analogous to the pause after exhalation during breathing. Swallowing is comparable to the active inhalation and exhalation phases of respiration. Every time one swallows the tongue should move like a wave going anterior to posterior against the entire palate. There is significant variability in the literature as to the amount of times a human swallows per day and seems to be dependent upon salivary flow rate and volume whereas a higher flow rate/smaller volume correlates with more frequent swallowing and a slower flow rate/higher volume correlates with less frequent swallowing patterns. One study found a range between 18-400 times per hour (Rudney, Ji, & Larson, 1995). The Academy of Orofacial Myofunctional Therapy cites that humans swallow 500-1000 times per day. According to Orofacialmyology.com, citing the works of Flanagan and colleagues, adults swallow about 585 times per day and children 800-1000. Swallowing creates a force directed up against the palate. One study, (Mcglone & Proffit, 1973), found tongue to canine and molar contact ranged from around 150-200 g/cm2 which is around 1 lb/in2. This is in contrast to other sources such as Wikipedia (Tongue Thrust) claiming 4 pounds of pressure is created during a swallow. However, whether the force is 1 or 4 lbs there is still a rhythmical pressure directed against the maxilla and sphenoid facilitating cranial flexion and extension movement which can potentially assist with cerebrospinal fluid motility. Therefore, the tongue may be referred to as “the cranial diaphragm” and serves to “pump” the cranium.
Posturing with the tongue on the palate provides a “brake” to the cranial system against asymmetrical neuromuscular torque. Because the right respiratory diaphragm is thicker, has broader spinal attachments, and is thus more powerful than the left, there is a tendency for these spinal attachments (~T8-L3) to orient to the right. In order to restore a forward facing position,the segments above T8 will attempt to compensatorily re-orient to a forward facing direction by moving left. This creates an increased anterior lower left rib flare (external rotation) relative to the right. Furthermore, the torsion imparted to the postural system by the asymmetrical diaphragm imposes ascending forces throughout the chain potentially facilitating craniofacial asymmetry. It has been shown in the normal population that almost 75% of cranial strain patterns exist with a propensity for the sphenoid to be elevated on the right. (Timoshkin & Sandhouse, 2008). As a result of the diaphragm’s torque, the left diaphragm leaflet’s zone of apposition is reduced relative to the right. By restoring the left diaphragm leaflet’s zone of apposition there will be a reduction in torsion going through the postural system. Similarly, just as restoring more symmetrical apposition of the diaphragm against the rib cage will reduce postural torque, the tongue in apposition to the palate serves to anchor the maxilla against torsional forces around it. Without this opposing force, craniofacial asymmetry and potential dysfunction may be more likely to occur.
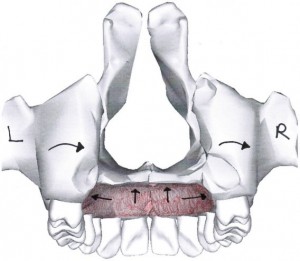
Braking effect of the tongue against maxilla torque. Adapted from https://commons.wikimedia.org/wiki/File:Maxilla_close-up_posterior.png
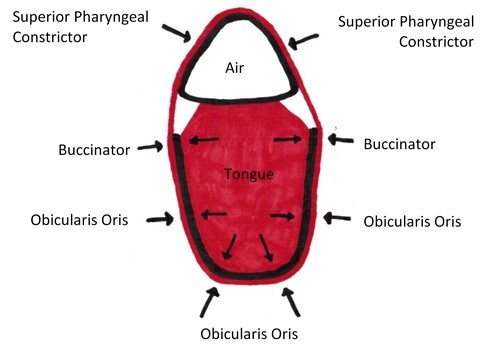
A cranial zone of apposition of the tongue against the palate provides a steady expansion force against the maxilla to maintain the size of the dental arch and prevent malocclusion. Without this consistent pressure, the arch can be overpowered by the opposing constricting forces imparted by the orbicularis oris, buccinator, and mentalis.
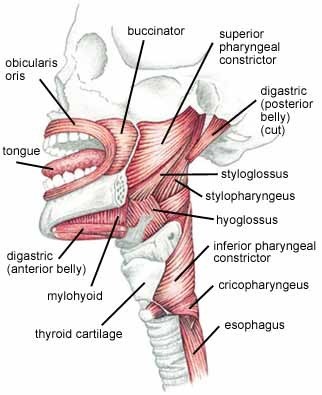
The orbicularis oris, buccinators, and superior pharyngeal constrictor form a neuromuscular sling around not only the arch but also the airway. Descending from the superior pharyngeal constrictor, the sling continues around the airway via the middle and inferior pharyngeal constrictor. Therefore, over facilitation within this neuromuscular chain can not only constrict the dental arch but also the airway.
A narrow arch may not allow enough room for the teeth and can thus facilitate crowding and malocclusion problems. This will be particularly problematic if there is a reverse swallow pattern characterized by thrusting the tongue forward into the front teeth and sucking the cheeks and/or lips inward via buccinator, obicularis oris, and/or mentalis hyperactivity. This type of swallow pattern occurs during infancy as a primitive reflex to facilitate breastfeeding behavior but could potentially not become integrated during development to allow an adult physiologic swallow. During normal swallowing the facial muscles should be relaxed with the tongue moving upward against the palate. If breastfeeding is ceased too early and soft foods introduced too soon it can possibly maintain a tongue thrust pattern. Anterior tongue thrusting can also lead to an anterior open bite due to the repetitive forces against the front teeth coupled with the tongue habitually resting against the incisors which typically accompanies this motor pattern. It is also common for the tongue to be resting on the mandibular teeth and in between the arches with a reverse swallow behavior impacting proper eruption and occlusal relationships of the teeth. Malocclusion is associated with imbalanced cervical-cranial-facial posture such as TMJ dysfunction, neck pain, and headaches. After every swallow the teeth should lightly come into occlusion. If a malocclusion exists with interferences, this can further contribute to postural problems. (Adhikari, Kapoor, Prakash, & Srivastava, 2011)
An habitual tongue and palate interface helps direct growth and development of the craniofacial system. The tongue in apposition to the palate provides a mechanical stimulus that is interpreted at the cellular level to promote osseous growth. (Singh, G. D. and Krumholtz, 2009). The force of the tongue against the floor of the maxilla guides its growth in a forward, upward and outward direction. Without this mechanical stimulus, the maxilla may have reduced spatial dimensions in these directions setting up a template for a narrow and elongated facial structure.
Oromotor activity facilitates craniofacial development. In utero, the embryo begins to establish craniofacial sensory-motor relationships in conjunction with embryological growth and differentiation. Postnatally, the latch and suck functions during breastfeeding promote the coupling of tongue-on-palate posture and oromotor function with concurrent nasally breathing. The tongue in apposition to the palate provides a neurosensory stimulus for orofacial motor learning. Furthermore, due to the anatomical brainstem proximity of the vagus nerve nuclei positioned next to the afferent and efferent craniofacial nerve nuclei there is cross-talk between craniofacial and autonomic nervous system processing. The vagus nerve even innervates the palatoglossus muscle, which functions to elevate the tongue towards the posterior soft palate. Activity in one nuclei can stimulate the other and vice versa. Likewise, reduced signaling in afferent and efferent craniofacial nuclei can impact vagal tone and the reverse. Stephen Porges discusses in The Polyvagal Theory (Porges, 2011) the integrated nature of craniofacial motor-sensory function and autonomic nervous system regulation which ultimately allows for higher level cortical-directed behavior such as social engagement, executive functioning, and advanced motor-sensory integration to occur and develop. In other words, without a solid foundation of brainstem cranial nerve networks, patterns, and regulation it will be challenging for the cortex to integrate primitive reflex patterns and allow more complex sensory-motor skill acquisition.
Inadequate osseous development of the cranium and face has a number of potential consequences. The maxilla houses the floor of the nasal sinus cavities and if not developed adequately from an anterior and/or lateral perspective has the potential to restrict nasal airflow. A lack of nasal cavity space can also lead to deviated septums as the septum may collapse in on itself due to inadequate room. Turbinates and adenoids may become enlarged due to the increased turbulent airflow that occurs with a reduced size nasal cavity which only serves to further occlude the nasopharygeal space. Due to these restrictions in nasal breathing, mouth breathing may become the preferred route. As was previously discussed, mouth breathing is associated with a number of negative postural repercussions.
A lack of adequate anterior and/or lateral maxillary growth will not allow enough room for the dentition. Narrowed and retruded maxillae are prone to crowding and malocclusion. An underdeveloped maxilla has the potential to create either a Class III or II malocclusion relationship with the mandibular teeth as well. As was previously discussed, malocclusion correlates to postural dysfunction.
There is a paucity of research regarding “ideal” and “normal” resting position of the tongue. This may be due in part to the challenges of imaging it in real time situations without providing altered sensory input that would bias the results. One study compared tongue position between non-cleft palate, unilateral cleft palate, and bilateral cleft palate individuals (McKee, 1956). It found that the apex of the tongue tended to rest on the lingual surface of the mandibular incisors and not on the palate while the posterior dorsum of the tongue rested against the uvula and posterior soft palate. It was also determined that there was increased convexity and higher static and dynamic tongue posture of the non-cleft subjects while the bilateral cleft subjects demonstrated the lowest position. In another study on edentulous (without teeth) (Kotsiomiti, Farmakis, & Kapari, 2005) individuals, it was determined that in the majority of the edentulous cases the tongue rested in the lower aspect of the mouth. Lee et al (Lee, Chen, Lee, & Chang, 2009) noted that improved denture retention occurred with improved tongue posture. The International Association of Facial Growth Guidance (Orthotropics) led by Drs. John and Mike Mew advocate that the tongue is meant to rest in full contact against the entire palate which includes the hard and posterior soft palate. This clinician supports this position and finds that the best postural outcomes are associated with the ability to achieve this tongue and palatal relationship.
The most common pattern this clinician sees in practice is individuals with either no habitual contact with their tongue on their palate with the tongue resting between the upper and lower arches or only contact the anterior 1/3 with or without pressure against the front teeth. In agreement with Dr. Mew, (Mew, 2013) the tongue exerts its most beneficial physiologic function when resting against the entire palate which can be divided into thirds. The first 1/3 (alveolar ridge) is immediately behind the upper front teeth, angles upward, and sometimes has texture. The next 1/3 is the topmost and hardest part of the palate followed by the soft downward sloping posterior 1/3rd. Individuals who have never habitually postured in this manner may have trouble sensing and knowing proprioceptively where their tongue is in relation to their palate. Some may have never felt this before in their life. The reason behind this lack of tongue/palate contact in many cases is due to a lack of flexibility of the tongue to adequately contact the entire palate. This is usually due to a tongue tie, formally referred to as ankyloglossia.
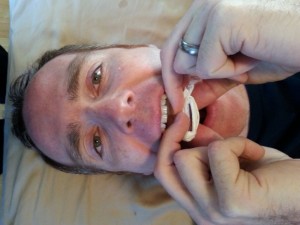
Ankyloglossia is an abnormal frenulum restricting tongue movement. The frenulum may be excessively short, thick, and/or tight displaying varying degrees of anatomy. Normally during development the frenulum helps to facilitate forward growth of the tongue but should undergo apoptosis and disappear to allow full mobility of the tongue at birth. Tongue ties are usually also associated with lip ties (frenulum restriction between the upper and/or lower lip and gum). During development either of these ties can negatively impact breastfeeding and speech production. Tongue and lip ties have genetic and environmental etiologies to be discussed in the latter part of this article. The current incidence is estimated to be 4-10% of newborns but it is thought that this is an under-representation due to lack of awareness of the condition and a consistent established criteria in diagnosis. (Rowan-legg & Society, 2015) They are more common in males than females (~4/1 ratio). (Tow et al., 2014)
If a tongue tie exists and is restricting full palatal contact, then tongue “stretching” or inhibition of the tongue retruders and depressors is recommended. This can be easily instructed for “self application” by the patient as follows:
“Wrap a napkin or paper towel (something with relative thickness and texture) around the tongue, close to the front base of the tongue. You may feel a fibrous, central, tendonous, tissue here called your frenulum. Grip your fingers around your tongue with the thumbs at the frenulum and gently pull the tongue up towards your hard and posterior soft palate (wherever your are restricted from contacting) so you feel a stretch. You can then direct the amount of force by opening and closing your jaw. You may also open and move the jaw side to side to get different fibers to stretch. Perform for 1-2 minutes and repeat frequently throughout the day. Laying on your back is typically the easiest position but it can also be done in sitting and standing positions. As you gradually begin to gain improved tongue to palatal contact you need to develop the habit of keeping it there at all times except for eating, drinking, or talking. It can take weeks to develop the habit of keeping your tongue positioned on the palate. It is recommended to leave reminders out to cue you as your brain learns this new postural pattern. “
As an individual learns to achieve a tongue/palatal zone of apposition at rest, it is also beneficial to retrain other dynamic craniofacial neurosensory functions. These include the ability to move the jaw in all directions while maintaining tongue/palatal contact, proper swallowing, and lip seal. It can also involve moving the eyes in conjunction with the jaw in different directions which can further be superimposed on various postural patterns of the rest of the body. As previously described, enhancing the brainstem cranial nerve pathways will promote higher level cortical sensory-motor integration and learning.
If one is still not able to achieve a proper tongue/palate relationship after diligent stretching then consideration of getting the tongue tie “clipped” to free up the tongue may be appropriate. This is usually done via laser but can also be accomplished with liquid nitrogen or scalpel surgical intervention. Dentists and ENTs typically perform the procedure. Clinically, this clinician finds that stretching is effective in most cases and this procedure does not need to be done very often. Tongue tie is particularly important for identification in newborns as the tongue restriction may serve as a barrier for breastfeeding (Rowan-legg & Society, 2015). It is encouraged that each newborn be screened for tongue tie at birth to ensure successful breastfeeding capability which is essential to spark the craniofacial osseous and sensory-motor developmental cascade.
After restoration of proper tongue-on-palate posture an individual may still have persistent breathing and postural dysfunction, oromotor problems (i.e., lip seal, tongue thrusting, and bruxism), and/or malocclusion that is contributing to pain and functional limitations. In such cases, consideration of a maxillary expansion appliance such as an ALF (Alternative Lightwire Functional Appliance) (Bronson, Bronson, & Holway, 2015) or Orthotropics (Mew, 2013) coupled with orthodontia and orofacial myofunctional training may be appropriate. Nasal surgery could be indicated as well. These appliances create a neurosensory expansion stimulus across the palate, similar to what the tongue would apply, but at a stronger force. The result is all of the above benefits that were previously described for posturing the tongue on the palate and initiation of a process called palatal and circumaxillary osteogenesis. Even in adults, it is possible to stimulate growth of the stem cells along the sutures of the cranium and expand not only the palate but the circumaxillary sutures as well thus opening up the craniofacial complex. Epigenetic Orthodontics by Dr. Dave Singh explains and provides evidence for this mechanism. The end result of this facial expansion is increased space throughout the cranium including the nasal cavity and sinuses to allow improved airflow, maxilla to mandibular relationships, increased dental arch space to accommodate the teeth, address overcrowding issues, and increased room for the tongue on the palate. In addition to the spatial improvements, the craniofacial sensory-motor system may be facilitated to reorganize and integrate primitive reflex patterns such as tongue thrusting to allow higher level cortical-directed oromotor function. All of these factors can facilitate improved breathing and neuromuscular capacity of the cervical and craniofacial system.
Dietary Links to Craniofacial Development
Why is there an increasing prevalence of tongue ties, tongue thrusting, narrow and restricted dental arches with associated malocclusion, and/or occluded nasal and oropharyngeal airways? The reasons are widespread in addition to interacting with one another. First, there have been dramatic changes in our diet over the past few hundred years with regards to nutritional content and consistency yet our genetic blueprint has not significantly changed. Studies show that starting around 10,000 years ago during the agricultural revolution deterioration began within the craniomandibular system.(Boyd, 2011, 2012) Epigenetic and microbiome changes are also a prominent component of these findings as are environmental factors. In addition, feeding (bottle vs breastfeeding) and birthing changes (vaginal vs. cesarean which influences an infant’s developing microbiome) have also had an impact on craniofacial development and function. (Tow et al., 2014) Jennifer Tow is a holistic lactation specialist and provides numerous webinars on these topics which can be accessed here: http://holisticibclc.blogspot.com/. The remainder of this article will focus on some of these dietary and interacting epigenetic factors.
Since the introduction of refined sugar about 400 years ago, the onset of the industrial revolution in the 1800s, increased food processing technologies further facilitated by World War II, and the creation of massive food production giants during the 20th century there has been a dramatic change in the human diet within a very short time period relative to our existence. The most significant aspects of these dietary shifts are extreme increased consumption of sugar, processed foods, pasteurized dairy, GMOs, pesticide-laden and nitrogen-fertilized fixed produce, and animal meat that had been fed a grain-based diet while being injected with anti-biotics and hormones. This is contrast to the hunter-gatherer diet we consumed for ~95% of our modern human existence.
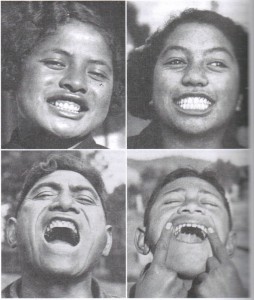
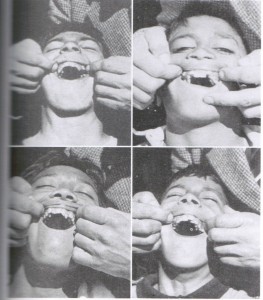
Weston Price was a dentist who noticed a decline in his patients’ dental health over his years of practice during the early part of the 20th century. He particularly saw an increase in tooth decay coupled with changes in facial structure such as narrower palates and malocclusion. His retirement consisted of traveling around the world and investigating the craniofacial structure, health, and nutrition of cultures who were still consuming their indigenous diets. He wrote about his findings in Nutrition and Physical Degeneration (Price, 2009). Among all of the cultures he studied still eating a diet unaltered by commercialized modern foods he found that cavities, malocclusion, and craniofacial deficiencies were virtually non-existent. Whenever any members of these societies were exposed to modern processed and sugar laden diets they quickly began to deteriorate physically. The first generation in these cultures exposed to the modern diet displayed tooth and gum decay. Later generations displayed malocclusion and reduced development of the craniofacial system. The following pictures are from Price’s Nutrition and Physical Degeneration.
Dr. Price’s analysis of the diets of these indigenous cultures around the world showed variety with regards to macronutrient content as well as the amount of plant and animal food consumed. There was not a single culture that did not eat some sort of animal product (including insects). Furthermore, four times more calcium and 10 times more fat soluble (A, D, E, and K) vitamins were consumed compared to the standard American diet at the time (1930-40s). He also discusses the negative consequences of soil depletion of nutrients as a factor in the deficiencies.
In Nutrition and Physical Degeneration Price describes an association of mental retardation with underdevelopment of the middle 1/3 of the face and how this can compress the pituitary gland with correlating clinical effects. Price describes one of his patients (16 years old) with mental retardation and significant underdevelopment of the maxilla, an entirely occluded L nostril, and reduced opening on the right. He slept supine with his coat rolled under his neck and head maximally posteriorly rotated to facilitate mouth and airway opening. If he wasn’t in this position, he would awaken due to apnea type episodes. When this individual’s palate was surgically widened and then supported with a palatal expansion appliance (increased 1/2 inch over 30 days with radiographic evidence of new bone growth within the palate) there was a significant change in physical development and behavior. Price reports he began to grow facial hair; grew 3 inches within 4 months; his genitals grew from child to adult size within 12 weeks; and there was marked improvement in his cognitive and social behaviors. Furthermore, if he didn’t wear the appliance to maintain the widened cranial position he would revert back to his former behavior. Reinstating the appliance would once again result in improved function. This case supports a connection between pituitary function and craniofacial space.
Price describes how Dr. Hector Mortimer at McGill University looked at 3000 human retrospective cases and concluded there to be a direct link between facial and dental arch formation with pituitary function. As part of his research, Mortimer surgically removed the pituitary gland in young rats which then produced skull defects including a lack of forward face development, narrow nose, and arch. Furthermore, by giving them pituitary extracts he was then able to prevent these defects. He also correlated a vitamin E deficiency with these presentations. This provides support to the connection between pituitary function and craniofacial space and that improved pituitary function can lead to craniofacial development during growth years. This was in contrast to the former described case where it appeared that the reduced size of the cranium negatively impacted pituitary function and that a reversal of pituitary function was achieved via an expansion of the cranium. It seems likely that both can influence the other and that there are other potential nutritional factors involved such as fat soluble vitamin and/or mineral deficiencies.
In addition to a reduced size of the middle 1/3 of the face, Price discusses how an overall lengthening and narrowing of the skeletal structure tends to coincide with this craniofacial and physical degeneration phenomenon. A narrower pelvis has significant negative implications for the ability to give birth while a narrower rib cage has negative respiratory implications. Price describes a correlation between TB susceptibility and the structure of the rib cage stating that a chest that was narrow and deep was more prevalent in children who were lighter, had narrower nostrils, and more prone to TB.
Francis Pottenger, MD conducted nutrition studies on over 900 cats during the 1930-40s. (Pottenger, 1945, 2012) He fed the experimental group 2/3 cooked meat, 1/3 raw milk, and cod liver oil (vitamin A source) while the control group ate 2/3 raw meat, 1/3 raw milk, and cod liver oil. The results showed that the cats who ate raw meat weighed more, had a healthier coat, were not vulnerable to disease, and showed uniformity in structure and size. Meanwhile, the cats who ate cooked meat were lethargic, had cases of arthritis, decreased coordination, and dental degeneration. The offspring of the experimental group had smaller and flatter heads, narrower zygomatic arches, and nasal cavities. Furthermore, later generations fed a cooked-meat diet displayed even more pronounced developmental and health problems. These commonly included allergies, asthma, pronounced exhaustion, impaired coordination, gum abscesses, aggressiveness, nearsightedness, farsightedness, reproduction problems, increased rate of infection, leg bones that tended to increase in length, trabeculae becoming courser with less calcium present, thyroid problems (underactive or inflammatory), inflammation of the nervous system (paralysis, meningitis), heart and lung problems, and decreased visceral volume and size of the thoracic and abdominal cavities. These patterns of deterioration represent a multi-system impact such as immune, endocrine, visual, neuromuscular, behavioral, skeletal, respiratory, and cardiac. Furthermore, females displayed reduced fertility with increasing rates of miscarriage and death during labor, as well as decreased ability to lactate. In fact, the 3rd generation of cats who ate cooked meat were unable to reproduce as it was not possible to create a 4th generation.
Pottenger tried to regenerate the cats who ate cooked meat by switching them over to a completely raw food diet. When a female cat was subjected to a cooked meat diet for 12-18 months and then returned to a normal diet (3-4 years) her reproductive ability was significantly impacted to the point that she was unable to give birth to normal kittens. He ultimately found that it took 4 generations to reverse the problems and even then it wasn’t consistent amongst an entire litter.
Pottenger also compared pasteurized, condensed, and sweetened condensed milk (2/3 of total diet) in conjunction with 1/3 raw meat and cod liver oil. The results showed deterioration and dental problems with all experimental conditions compared to raw milk which progressively worsened from pasteurized, condensed, to sweetened condensed milk. In other words, the more sugar in the milk the poorer the outcomes.
The specific food selections in the Pottenger experiments may not necessarily be directly applicable to humans in that humans and cats have different nutritional requirements. For example, human vs cat consumption of cooked meat may not be as detrimental since cooking will destroy an enzyme essential to the development of cats, while this is not the case for humans. Nonetheless, these findings do show similar results (impaired craniofacial development and physical degeneration) to what Price found in humans undergoing dramatic dietary changes towards processed and sugar laden foods.
What the Price and Pottenger studies demonstrate is a correlation between nutritional intake, craniofacial development, as well as endocrine health. The generational propagation and exacerbation of the structural and physiological changes also indicate epigenetic mechanisms at work. This means that nutritional behaviors and their structural and physiological consequences in one generation can be passed on and actually exacerbated in subsequent generations. Furthermore, the findings of these investigations parallel what is happening in today’s modern society of which malocclusion and decreased development of the face, particularly the maxilla and mandible are common. There is also a parallel between the inflammatory and endocrine dysfunction found in Price’s and Pottenger’s work and these problem’s increasing prevalence in our society today.
Another significant dietary factor over the past century or so is the change in the consistency of our foods. Our modern diets mainly consist of soft foods while our evolutionary ancestors typically ate tougher foods which could facilitate more facial muscle strength. There is an association between increased vertical facial growth and facial muscle weakness. (Mew, 2013) However, it is unlikely that facial weakness associated with a lack of chewing challenge is the sole reason behind increased vertical facial growth. First, in Pottenger’s studies on raw vs. pasteurized/fortified milk there was no difference between the consistency of foods between groups yet the outcomes demonstrated the same patterns in craniofacial structure as the raw vs cooked meat groups. Second, it is questionable as to how much of a difference there was in masticatory challenge between the raw vs cooked meat groups. Third, Pottenger’s human studies comparing different milk products exhibited no differences in food consistency and still showed the same craniofacial developmental patterns. It is possible that the inherent nutritional differences between ancestral diets that happen to contain tougher foods vs less nutritional modern foods that are also softer in consistency may be influencing these developmental patterns. Furthermore, one must also consider that modern babies are typically weaned from breastfeeding and introduced to soft foods earlier than primitive infants.
Connecting Nutritional Deficiencies with Genetic Expression of Craniofacial Development
There are genetic correlations related to tongue ties and cleft palate such as the TBX22 gene mutation (Andreou et al., 2007). Cleft palate and tongue tie are also associated with the MTHFR (methylenetetrahydrofolate reductase) enzyme mutation. This type of mutation is called a SNP (single nucleotide polymorphism) and involves a single nucleotide swap in the DNA sequence (example-thymine is used instead of cytosine). Dr. Benjamin Lynch is a leading expert on the MTHFR mutation and has provided many of the references below for this information. His website MTHFR.net is an excellent source of the latest information on this topic.
There are over a million different potential SNPs which have been around for much of humanity with some more significant than others related to health. The MTHFR SNP has specific implications for craniofacial development. This enzyme is a key component to metabolizing folate which is a crucial element of the methylation process. Methylation is the process of adding methyl groups (carbon attached to 3 hydrogens) to various compounds (including DNA) which are needed for proper function of: turning on and off genes (gene regulation-epigenetics); processing chemicals including endogenous and xenobiotic compounds (detoxification); building and metabolizing neurotransmitters; processing hormones; building immune cells; DNA and histone synthesis; producing energy; myelination; and building and maintaining cell membranes. These functions are vital to human regulation.
There are two variants of the MTHFR mutation: MTHFR C677T and MTHFR A1298C with different potential combinations. For example, each individual has 2 alleles of the gene (one from each parent). It is therefore possible to have two normal genes, one normal gene in combination with either the C677T or the A1298C gene, two C677T genes, two 1298C genes, or one C677T and one A1298C gene. Depending on the combination present, this will tend to correlate with different levels of reduced enzyme function ranging from a 20% -75% reduction. (van der Put et al., 1998)
Folic acid deficiency is associated with midline developmental problems such as neural tube defects, cleft palate, and tongue tie. (Rooij et al., 2003) The MTHFR SNP has a significant impact on the utilization of folic acid. The MTHFR enzyme is needed to convert folic acid into its bioavailable form, 5-Methyltetrahydrofolate (5-methyl-THF). Synthetic folic acid, which is what is typically given in supplements (particularly pre-natal) and in fortified foods, is not a readily useable form of folic acid and is difficult to convert into the bioavailable form. (Mitchell, Murray, Brien, & Christensen, 2003). If the MTHFR mutation exists one can be further challenged with this conversion. Furthermore, it has been shown that high levels of synthetic folic acid compete with the bioavailable form at receptor sites further inhibiting bioavailable folate uptake. (Rooij et al., 2003) Elevated levels of synthetic folic acid may also desensitize folate receptors downregulating them and making it even more difficult to metabolize folate. (Henderson, 1990; Houghton, Yang, & O’Connor, 2009) Therefore, it is important (especially in cases of the MTHFR mutation) to supplement with the bioavailable forms of folate which include: L-5-MTHF = L-5-Methyltetrahydrofolate = 6(S)-L-MTHF = 6(S)-L-Methyltetrahydrofolate or L-Methylfolate Calcium = Metafolin = Levomefolic Acid. Seeking Health, Biotics Research Laboratories, and Orthomolecular are examples of supplement companies that carry these types of bioavailable folate. One may take caution with labels such as: 5-MTHF; 5-Methylfolate; and 5-Methyltetrahydrofolate. These forms may not be the L or 6(S) forms which are the biologically active forms. (Lynch, 2012) It is best to contact the maker of the supplement to verify the forms.
Another SNP, BCMO1, is associated with a 60% reduced ability to convert beta carotene to retinol, the bioactive form of vitamin A. “Higher levels of vitamin A intake from multivitamins and liver sources also seemed to protect against cleft palate indicating that adequate levels of vitamin A may be required for normal development of the primary palate.” (Mitchell et al., 2003) Dr. Price also found an association of vitamin A deficiency with cleft palate.
It is important to note that folate metabolism and the methylation cycle are dependent on other nutrients that are important to obtain via diet and/or bioavailable supplementation such as B12 (methylcobalamin); B6 (Pyridoxine); B2 (Riboflavin); Betaine; Choline; and Cysteine. Furthermore, there are many other interrelated metabolic pathways requiring their own plethora of nutrients that are integrated with the methylation cycle and gene expression regulation. Other types of SNPs within the methylation cycle may also potentially effect these processes requiring specific nutrient supplementation. Therefore, when considering dietary and nutritional needs these must all be considered. Other variables of significance include digestion (which can further be broken down into stomach, pancreatic, gallbladder, small intestine, and large intestine function); blood sugar regulation; endocrine and immune function; ANS and CNS regulation; toxicity levels and detoxification capacity; and any number of potential environmental stressors.
Despite the millions of potential SNPs that may be present in one’s genome and thus potentially be expressed, we still have the capability to influence their full expression and thus impact on our system’s physiologic regulation and developmental patterns. It has been shown that genetics account for 20-50% of cleft palate with “the remainder associated with a wide variety of environmental factors during early pregnancy, such as smoking, use of alcohol and chemotherapeutic drugs, lack of maternal nutritional supplements such as folic acid or other vitamins, viral infection, and exposure to agricultural chemicals or other teratogens. More complex factors have also been implicated, including maternal age, low socioeconomic status, psychological stress in the mother, altitude, and conditions of hypoxia, in some cases with good supporting evidence provided by animal models.” (Andreou et al., 2007) An example of this genetic-environmental interaction can be demonstrated in Italy where there is a high prevalence of the MTHFR mutation yet a low incidence of neural tube defects likely due to dietary and environmental factors. This is in contract to Mexico and China where there is a high rate of the MTHFR mutation as well as neural tube defects which is likely explained by environmental and/or dietary challenges. (Yang et al., 2013) (Wilcken et al., 2003)
In conclusion, cervical-cranial-facial postural function is integrated with various structural and sensory-motor aspects of the craniofacial system, in particular the relationship between the tongue and palate. Restrictions of the tongue (ankyloglossia) can have a detrimental effect on craniofacial growth and thus the postural system. Ankyloglossia and craniofacial development have correlations to nutritional deficiencies interacting with genetic factors as well as other environmental and behavior variables. Prevention of tongue tie and craniofacial deficiency can be addressed via nutritional optimization which includes genetic and environmental interactions. Preconception, in-utero, and post-natal nutritional support is ideal to establish a foundation for optimal growth and development. Intervention of an already established tongue tie with accompanying craniofacial and postural dysfunction should be addressed as early as possible but can also be treated as an adult. Adults can significantly benefit from orofacial postural retraining interventions as well as have the ability to expand and modulate the craniofacial structural and sensory-motor system.
References
Adhikari, H. D., Kapoor, A. K., Prakash, U., & Srivastava, A. B. (2011). Electromyographic Pattern of Masticatory Muscles in Altered Dention Part II. Journal of Conservative Denstistry, 14(2), 120–127.
Andreou, A. M., Pauws, E., Jones, M. C., Singh, M. K., Bussen, M., Doudney, K., … Stanier, P. (2007). TBX22 Missense Mutations Found in Patients with X-Linked Cleft Palate Affect DNA Binding , Sumoylation , and Transcriptional Repression. The American Journal of Human Genetics, 81(October), 700–712. http://doi.org/10.1086/521033
Boyd, K. L. (2011). Darwinian Dentistry. Journal of American Orthodontic Society, 34–40.
Boyd, K. L. (2012). Darwinian Dentistry Part 2. Journal of American Orthodontic Society, 28–33.
Bronson, J. M., Bronson, J. A., & Holway, C. (2015). Introducing the Advanced Light Force ( ALF ) Appliance. Oral Health, 10–17.
Henderson, G. B. (1990). FOLATE-BINDING PROTEINS. Annual Review of Nutrition, 10, 319–335.
Houghton, L. A., Yang, J., & O’Connor, D. L. (2009). Unmetabolized folic acid and total folate concentrations in breast milk are unaffected by low-dose folate supplements. American Journal of Clinical Nutrition, 89(1), 216–220. http://doi.org/10.3945/ajcn.2008.26564
Kotsiomiti, E., Farmakis, N., & Kapari, D. (2005). Factors related to the resting tongue position among partially and completely edentulous subjects. Journal of Oral Rehabilitation, 32, 397–402.
Lee, J., Chen, J., Lee, H., & Chang, H. (2009). Improved denture retention in patients with retracted tongues. The Journal of the American Dental Association, 140(8), 987–991. http://doi.org/10.14219/jada.archive.2009.0308
Lynch, B. (2012). L-Methylfolate, Methylfolate, 5-MTHF, L-5-MTHF. What is the Difference!? Retrieved from http://mthfr.net/l-methylfolate-methylfolate-5-mthf/2012/04/05/
Mcglone, R. E., & Proffit, W. R. (1973). Patterns of Tongue Contact in Normal and Lisping Speakers. Journal of Speech and Hearing Research, 16, 456–473.
McKee, T. L. (1956). A cephalometric radiographic study of tongue position in individuals with cleft palate deformity.pdf.
Meiyappan, N., Tamizharasi, S., Senthilkumar, K. P., & Janardhanan, K. (2015). Natural Head Position: An Overview. Journal of Pharmacy and BioAllied Sciences, August(Suppl 2), S424–S427.
Mew, J. (2013). The Cause of Malocclusion. Broadfield.
Mitchell, L. E., Murray, J. C., Brien, S. O., & Christensen, K. (2003). Retinoic Acid Receptor Alpha Gene Variants , Multivitamin Use , and Liver Intake as Risk Factors for Oral Clefts : A Population-based Case-Control Study in Denmark , 1991 – 1994. American Journal of Epidemiology, 158(1), 69–76. http://doi.org/10.1093/aje/kwg102
Porges, S. (2011). The Polyvagal Theory: Neurophysiological Foundations of Emotions, Attachment, Communication, and Self-regulation.
Postural Restoration Institute. (2013). Retrieved from https://www.posturalrestoration.com/
Pottenger, F. (1945). Effect of Heat-Processed Foods and Metabolized Vitamin D Milk.
Pottenger, F. (2012). Pottenger’s Cats.
Price, W. A. (2009). Nutrition and Physical Degeneration.
Rooij, I. A. L. M. Van, Vermeij-keers, C., Kluijtmans, L. A. J., Ocké, M. C., Zielhuis, G. A., Goorhuis-brouwer, S. M., … Steegers-theunissen, R. P. M. (2003). Does the Interaction between Maternal Folate Intake and the Methylenetetrahydrofolate Reductase Polymorphisms Affect the Risk of Cleft Lip with or without Cleft Palate ? American Journal of Epidemiology, 157(7), 583–591. http://doi.org/10.1093/aje/kwg005
Rowan-legg, A., & Society, C. P. (2015). Ankyloglossia and breastfeeding. Canadian Paediatric Society, 20(4), 209–213.
Rudney, J. D., Ji, Z., & Larson, C. J. (1995). The prediction of saliva swallowing frequency in humans from estimates of salivary flow rate and the volume of saliva swallowed. Archives of Oral Biology, 40(6), 4–6.
Singh, G. D. and Krumholtz, J. (2009). Epigenetic Orthodontics.
Solow, B., Ovesen, J., Nielsen, P. W., Wildschiodtz, G., & Tallgren, A. (1993). Head posture in obstructive sleep apnoea. European Orthodontic Society, 15, 107–114.
Timoshkin, E. M., & Sandhouse, M. (2008). Retrospective study of cranial strain pattern prevalence in a healthy population. The Journal of the American Osteopathic Association, 108(January), 652–656.
Tow, J., Boyd, K., Lynch, B., Jacobson, H., Buckley, S., Jaminet, P., & Masterjohn, C. (2014). Tongue-tie, Epigenetics, and the Microbiome.
Uhlig, S. E., Marchesi, L. M., Duarte, H., & Araújo, M. T. M. (2015). Association between respiratory and postural adaptations and self-perception of school-aged children with mouth breathing in relation to their quality of life. Brazilian Journal of Physical Therapy, 19(3), 201–210.
van der Put, N. M. J., Gabreëls, F., Stevens, E. M. B., Smeitink, J. A. M., Trijbels, F. J. M., Eskes, T. K. A. B., … Blom, H. J. (1998). A Second Common Mutation in the Methylenetetrahydrofolate Reductase Gene: An Additional Risk Factor for Neural-Tube Defects? The American Journal of Human Genetics, 62(5), 1044–1051. http://doi.org/10.1086/301825
Wilcken, B., Bamforth, F., Li, Z., Zhu, H., Ritvanen, A., Redlund, M., … Botto, L. D. (2003). Geographical and ethnic variation of the 677C>T allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): findings from over 7000 newborns from 16 areas world wide. Journal of Medical Genetics, 40, 619–625.
Yang, B., Liu, Y., Li, Y., Fan, S., Zhi, X., Lu, X., … Sun, G. (2013). Geographical Distribution of MTHFR C677T, A1298C and MTRR A66G Gene Polymorphisms in China: Findings from 15357 Adults of Han Nationality. PLoS ONE, 8(3). http://doi.org/10.1371/journal.pone.0057917
Yeol, H., Jong, K., Jeong, I., Jung, H. D., Sohn, H., Duk, S., … Yun, S. (2013). Nasal Obstruction and Palate-Tongue Position on Sleep-Disordered Breathing. Clinical and Experimental Otohinolaryngology, 6(4), 226–230.